What Is the Placebo Effect? | Definition & Examples
The placebo effect is a phenomenon where people report real improvement after taking a fake or nonexistent treatment, called a placebo. Because the placebo can’t actually cure any condition, any beneficial effects reported are due to a person’s belief or expectation that their condition is being treated.
You feel that the pill relieves the symptoms, but at the end of the month you find out that you were given a placebo—and not the new medication. The perceived improvement you experienced was due to the placebo effect.
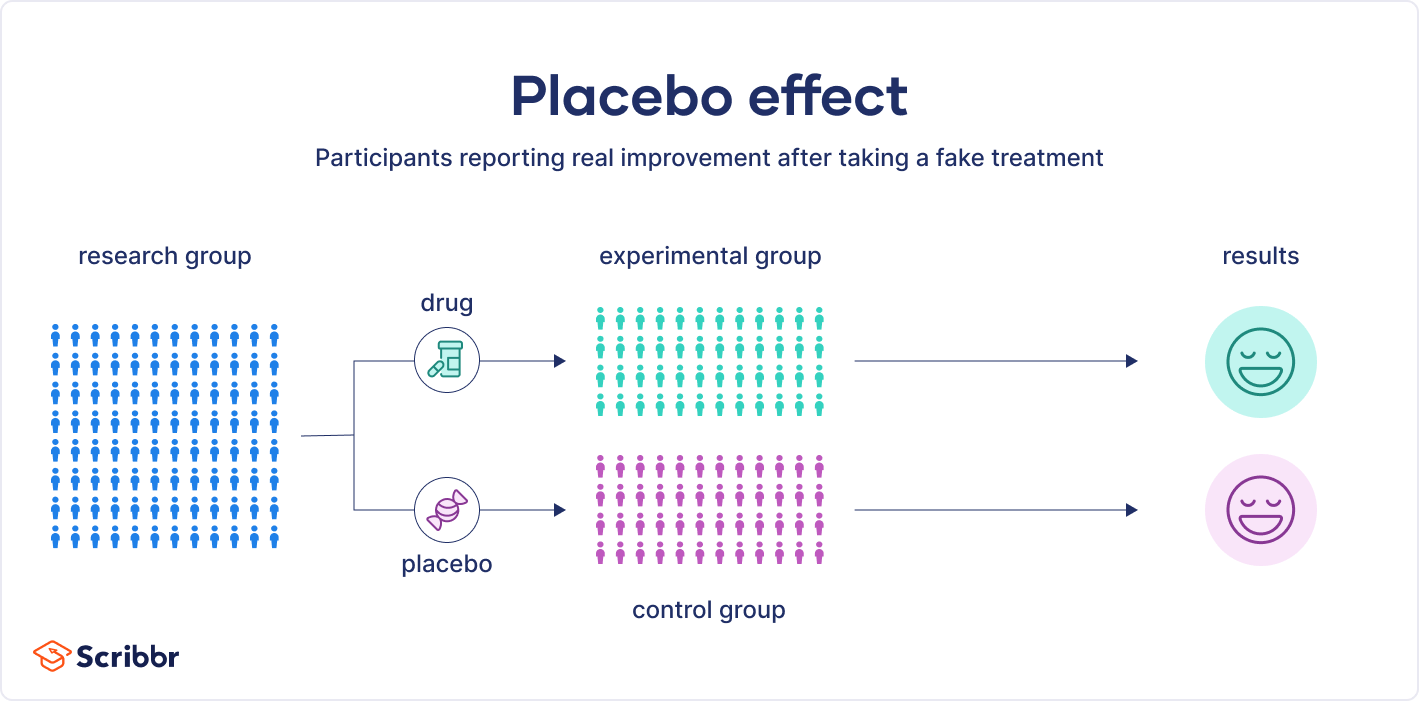
The placebo effect is often observed in experimental designs where participants are randomly assigned to either a control or treatment group.
What is a placebo?
A placebo can be a sugar pill, a salt water injection, or even a fake surgical procedure. In other words, a placebo has no therapeutic properties. Placebos are often used in medical research and clinical trials to help scientists evaluate the effects of new medications.
In these clinical trials, participants are randomly assigned to either the placebo or the experimental medication. Crucially, they are not aware of which treatment they receive. The results of the two groups are compared, to see whether they differ.
In double-blind studies, researchers also don’t know who received the actual treatment or the placebo. This is to prevent them from conveying demand characteristics to participants that could influence the study’s results. This is preferred over single-blind studies, where participants do not know which group they have been placed in, but researchers do.
Placebos may help relieve symptoms like pain, fatigue, or stress-related insomnia, but they don’t actually treat a condition or cure a disease. Note that due to ethical considerations, placebos are not always used in clinical trials. For example, as it would be unethical to leave terminal cancer patients untreated, placebos aren’t used in these types of studies.
What is the definition of placebo effect?
For some people, just the idea that they are taking medication makes them feel better. This occurs even if the medication is actually just a placebo. This phenomenon is known as the placebo effect. In other words, the perception of feeling better is triggered by the person’s belief in the benefit of the treatment.
When studying a new treatment, researchers must demonstrate that it is more effective than can just be explained by the placebo effect. To do so, they compare the results from those taking the new treatment with those from the placebo. In order to accurately compare the two groups, participants in clinical trials must not know whether they received the treatment or the placebo. If the two groups have the same reaction, the effectiveness of the new treatment is not supported.
Studies on so-called “open-label” or “honest” placebos, in which people are aware that they are being prescribed placebo medication for their condition, have shown that placebos can improve symptoms among people with irritable bowel syndrome or lower back pain. This suggests that people may experience the placebo effect regardless of whether they know that they are taking a placebo or not.
How does the placebo effect work?
Although the exact reasons for the positive effects of placebos are still being researched, a number of factors contribute to the phenomenon. These include:
- A person’s expectations or beliefs that they will get better. People who are motivated and expect their treatment to work are more likely to experience the placebo effect.
- The feeling of receiving attention and care due to participation in the study. This may reduce stress levels and trigger the body’s own pain-relieving chemicals.
- Classical conditioning, or the association people build over the years between a certain action, such as pill-taking, and positive results.
- A trusting relationship between doctors and patients or researchers and study participants from the sample. Listening to an expert you trust talk enthusiastically about a treatment can impact how you respond to it.
However, researchers do not attribute the placebo effect exclusively to psychology. A few other possible explanations include:
- Regression to the mean: When people first visit a doctor or start on a clinical trial, their symptoms might be particularly bad. But in the natural course of an illness, symptoms may subside on their own.
- Confirmation bias: Feelings of hopefulness about a new treatment may lead people to pay more attention to signs that they’re getting better and less attention to signs that they’re getting worse.
Placebo effect examples
The placebo effect illustrates how the mind can trigger changes in the body.
After participants take the pill, their blood pressure and pulse rate increases, and their reaction speeds are improved.
However, when the same people are given the same pill and told it will help them relax and sleep, they report experiencing relaxation instead.
If a person expects a treatment to do something, it’s possible that the body’s own chemistry can cause effects similar to what a medication might have caused. Additionally, researchers’ enthusiasm can contribute to the placebo effect.
The placebo effect can also explain the popularity of non-FDA-approved products.
Evidence from published studies show that it takes extremely high doses for CBD to be effective. Documented benefits of CBD in placebo-control trials require anywhere from hundreds to thousands of milligrams per day. This is the equivalent of taking almost an entire bottle each day, depending on the concentration.
Most people take 15 milligrams or less per day, far less than what the studies deem an effective dose. The placebo effect seems to play a role here: the expectation is so high that people start to believe it’s working.
Unless more research is conducted, there is no way to know for sure whether CBD products have real and measurable effects, or whether it’s the placebo effect that’s providing relief.
Downside of the placebo effect
The response of people assigned to the placebo control group may not always be positive. They may experience what is called a “nocebo effect,” or a negative outcome, when taking a placebo. The same explanation applies here. If you expect a negative outcome, it’s more likely you’ll have a negative outcome.
For example, in a clinical trial, participants who are given a placebo but are told what side effects the “treatment” may cause. They may have the same side effects as the participants who are given the active treatment, only because they expect them to occur.
Other types of research bias
Frequently asked questions about the placebo effect
- What causes the placebo effect?
-
Although there is no definite answer to what causes the placebo effect, researchers propose a number of explanations such as the power of suggestion, doctor-patient interaction, classical conditioning, etc.
- Why are placebos used in research?
-
Placebos are used in medical research for new medication or therapies, called clinical trials. In these trials some people are given a placebo, while others are given the new medication being tested.
The purpose is to determine how effective the new medication is: if it benefits people beyond a predefined threshold as compared to the placebo, it’s considered effective and not the result of a placebo effect.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Nikolopoulou, K. (2023, March 06). What Is the Placebo Effect? | Definition & Examples. Scribbr. Retrieved January 8, 2024, from https://www.scribbr.com/research-bias/placebo-effect/